PPT Subshells and Orbitals PowerPoint Presentation, free download ID6870753
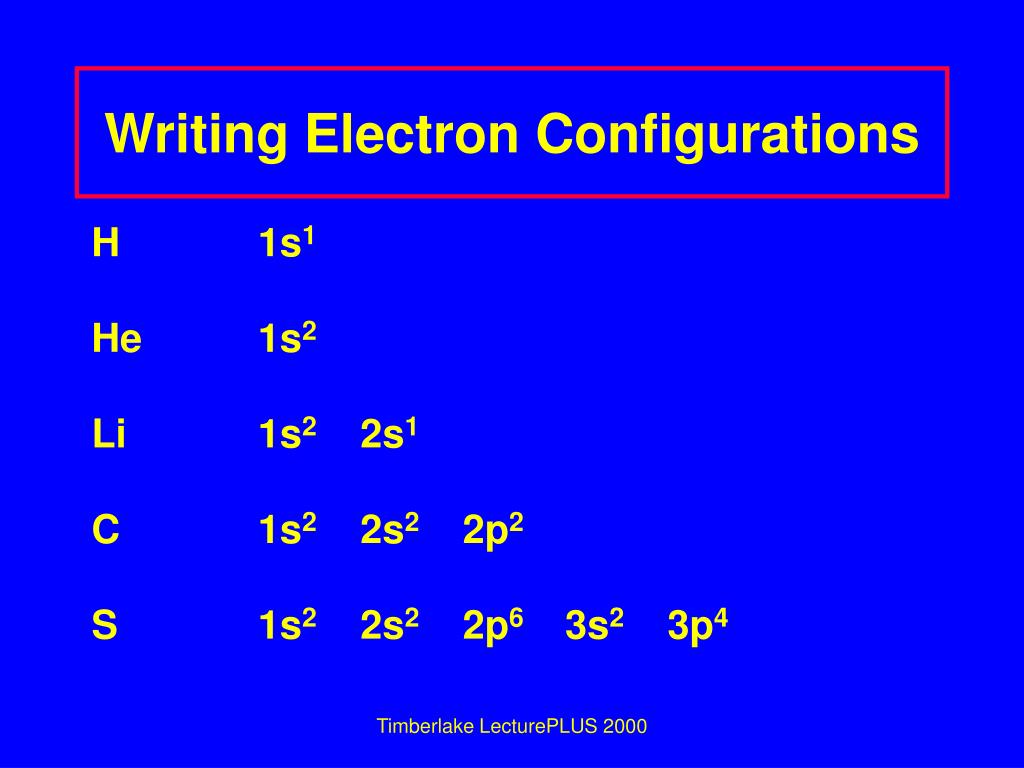
Based on the order of fill above, these 8 electrons would fill in the following order 1s, 2s and then 2p. So Oxygen's electron configuration would be O 1s 2 2s 2 2p 4. Special Cases. Configurations of ions present a special case of electron configuration and also demonstrate the reason for the formation of those ions in the first place.

How to find Protons & Electrons for the Sulfide ion (S 2) YouTube
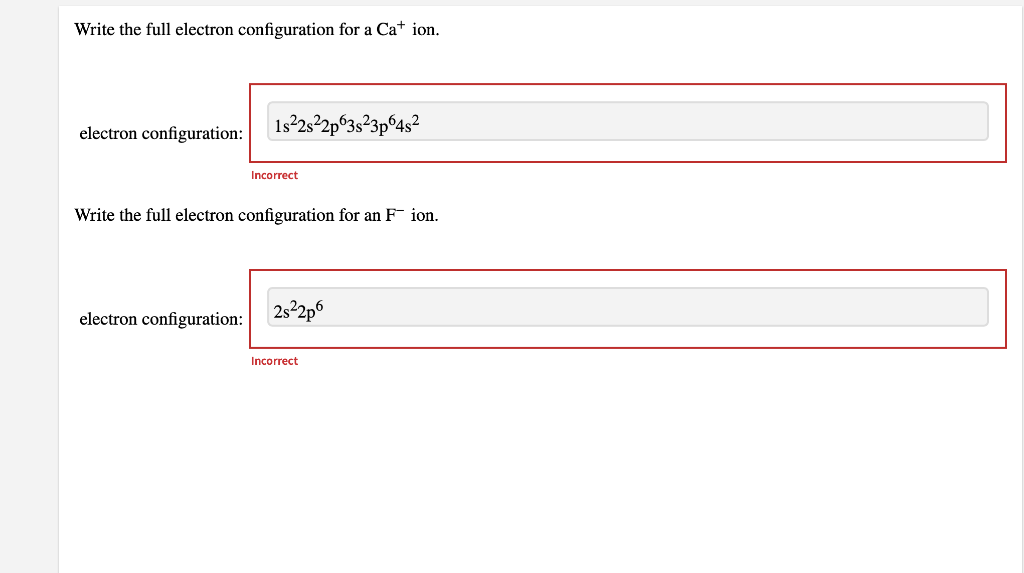
Mar 24, 2016 S2−:1s22s22p63s23p6 Explanation: A good starting point when looking for the electron configuration of an ion is the electron configuration of the neutral atom. In your case, the neutral atom is sulfur, S, which is located in period 3, group 16 of the periodic table.
Solved Write the full electron configuration for S2. full
Introduction to electron configurations Noble gas configuration Electron configurations for the first period Electron configurations for the second period Electron configurations for the third and fourth periods Electron configurations of the 3d transition metals Electron configurations Paramagnetism and diamagnetism The Aufbau principle

Electronic Configuration of Elements VanceknoeHampton
By extrapolation, we expect all the group 2 elements to have an ns2 electron configuration. Exercise 6.9.1 6.9. 1. Use the periodic table to predict the characteristic valence electron configuration of the halogens in group 17. Answer: All have an ns2np5 electron configuration, one electron short of a noble gas electron configuration.

chemistry How to find out unpaired electron in S2 molecule? Chemistry Stack Exchange
Electronic configuration: The arrangement of electrons into the orbitals of an atom using some fundamental principle is called its electronic configuration. Electronic configuration of Sulfur S: The electronic configuration of Sulfur is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 4. Electronic configuration of Sulfide S 2 -:

Electron Configuration Chart for the Elements Chart, Chemistry and Organic chemistry
AboutTranscript. Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Created by Sal Khan.

Which element has the electron configuration of 1s2 2s2 2p6 3s2 3p6? YouTube
What is the electron configuration of the sulfide ion ( S −2 )? Chemistry Electron Configuration Electron Configuration 1 Answer anor277 Nov 9, 2016 The sulfur atom has 6 valence electrons, and thus the S2− has 8 valence electrons. Explanation: So S2− is isolectronic with argon. Answer link

Electronic configuration Definition, Orbitals, & Facts Britannica
665 Share 113K views 4 years ago Electron Configurations In this video we will write the electron configuration for S 2-, the Sulfide ion. We'll also look at why Sulfur forms 2- ion and how.
S 2 Electron Configuration (Sulfide Ion) YouTube
The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number between 0 and 14. Thus in the building-up process for the lanthanoids.
Explain This Difference Based on Their Electron Configurations
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +12 m s = + 1 2 ).

Electronic Configurations Intro Chemistry LibreTexts
To help describe the appropriate notation for electron configuration, it is best to do so through example. For this example, we will use the iodine atom. There are two ways in which electron configuration can be written: I: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 5. or. I: [Kr]5s 2 4d 10 5p 5

2.2 Electron Configurations Chemistry LibreTexts
In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic table gives the following electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3.
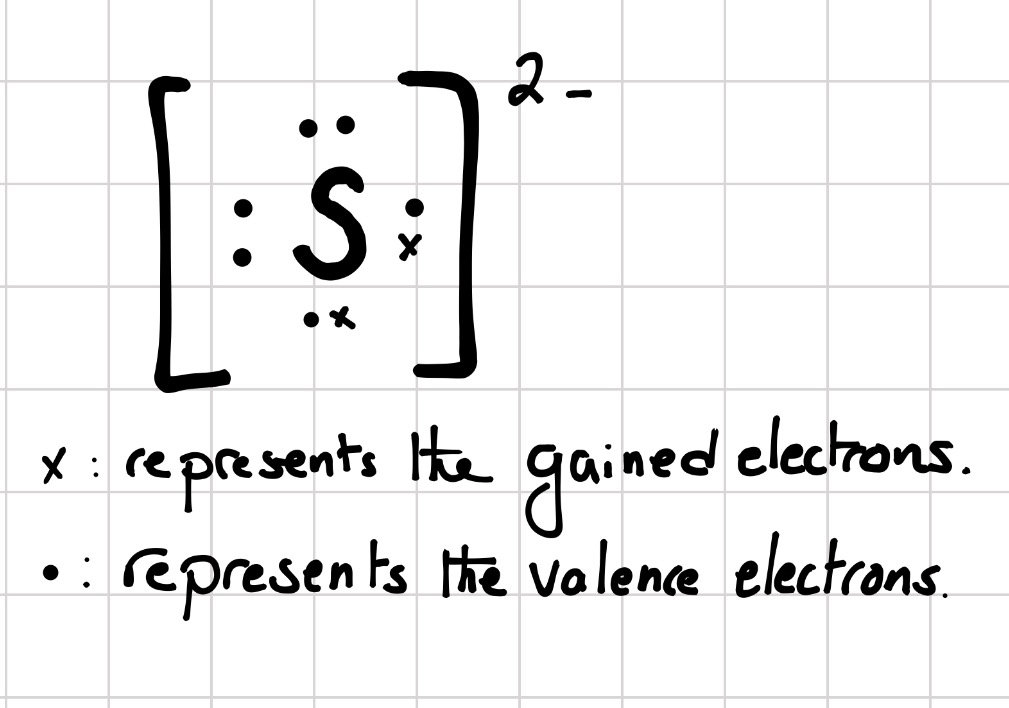
Which Lewis electrondot diagram is correct for a "S"^(2) ion? Socratic
PROBLEM 3.1.12 3.1. 12. In one area of Australia, the cattle did not thrive despite the presence of suitable forage. An investigation showed the cause to be the absence of sufficient cobalt in the soil. Cobalt forms cations in two oxidation states, Co 2+ and Co 3+. Write the electron structure of the two cations. Answer.

Which element has the electron configuration of 1s2 2s2 2p6 3s2 3p6 3d2 4s2 YouTube
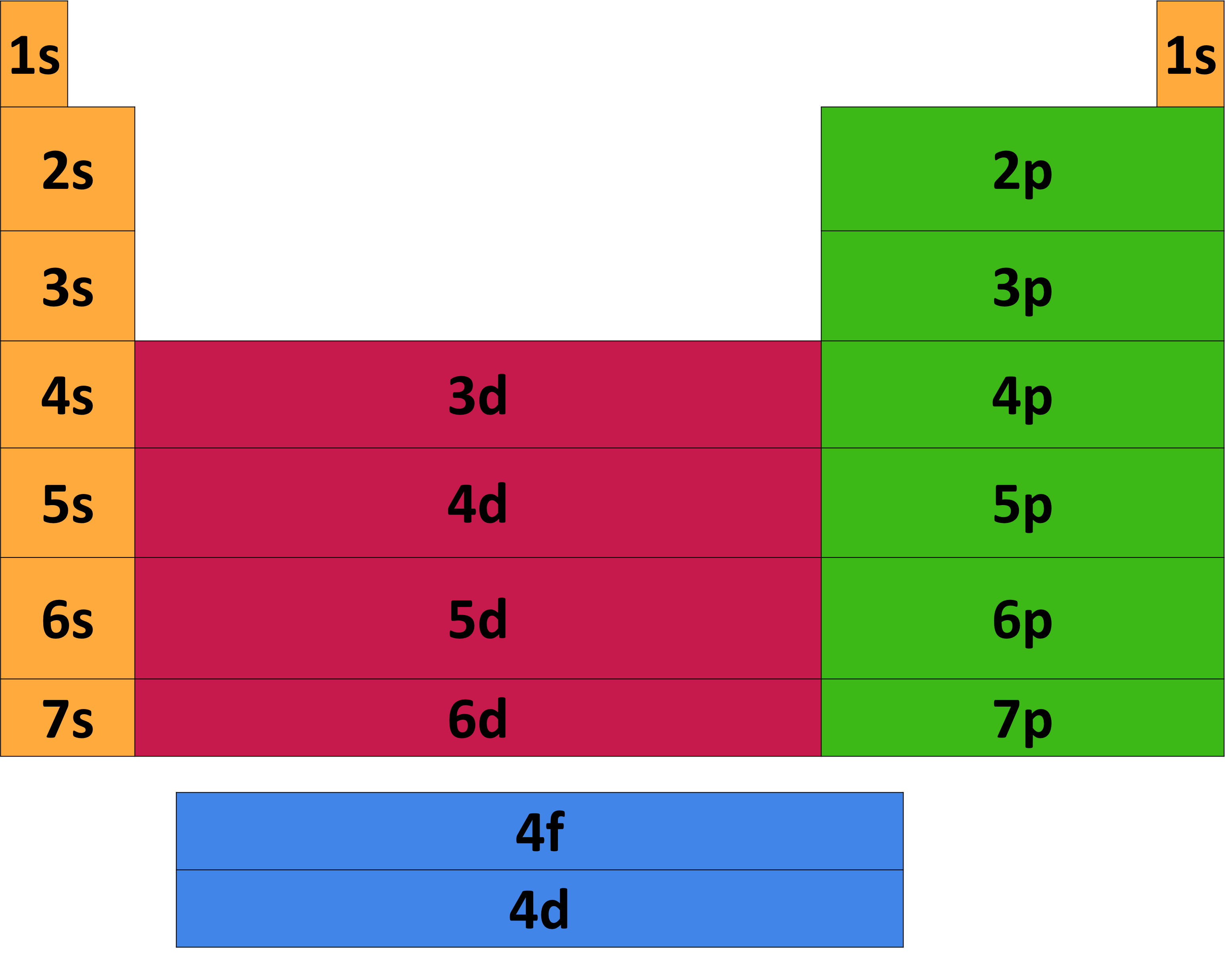
Key Questions How do electron configurations correspond to the periodic table? When looking at electron configuration, your fill order of electrons is: 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s etc. Group 1A (1), the alkali metals all end is s1. What period the element is in determines the 1st number.
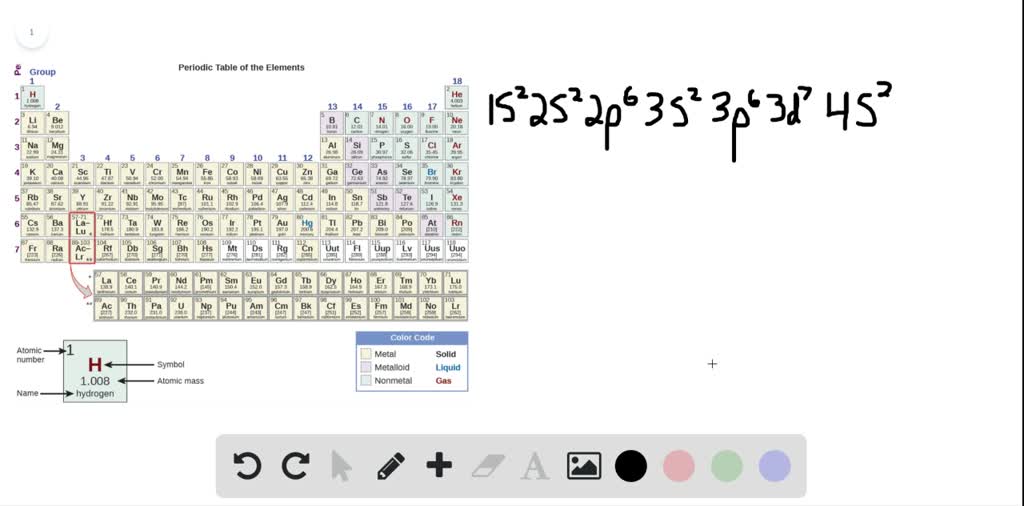
SOLVEDA neutral atom has the electron configuration 1 s^{2} 2 s^{2} 2 p^{6} 3 s^{2} 3 p^{3
In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for sulfur go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
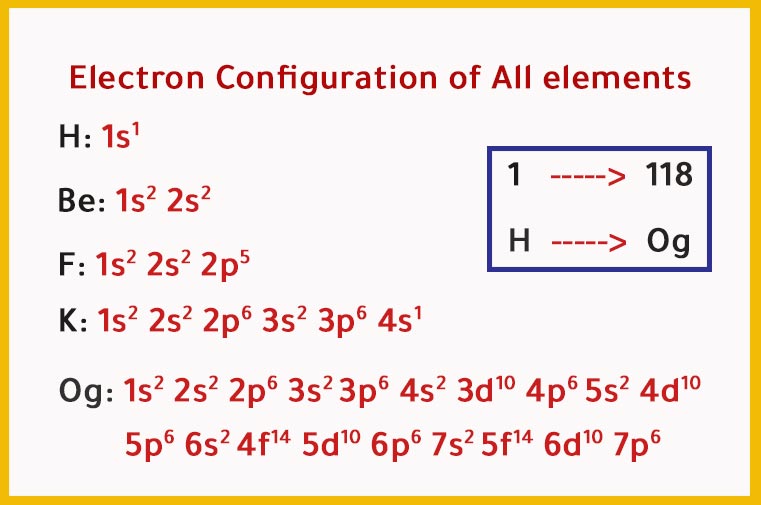
List of Electron Configurations of All elements 118
The arrangement of electrons in sulfur in specific rules in different orbits and orbitals is called the electron configuration of sulfur. The electron configuration of sulfur is [ Ne] 3s 2 3p 4 , if the electron arrangement is through orbitals. Electron configuration can be done in two ways. Electron configuration through orbit (Bohr principle)